Background: Racial and ethnic representativeness in clinical trials is a crucial step to mitigate disparities in outcomes. In 2017, the United States (US) Food and Drug Administration (FDA) issued a final ruling requiring trial sponsors to report race/ethnicity data to the clinicaltrials.gov registry. Black and Hispanic persons are underrepresented in clinical trials for several diseases, including hematologic malignancies (Hantel, Luskin et al. 2021). However, the reporting and representation of race and ethnicity for hemophilia interventional trials is unknown. Congenital Hemophilia is a rare bleeding disorder for which there have been significant treatment advances leading to substantial gains in life expectancy and improvements in quality of life for those with access to treatment. The aims of the current study are to assess 1) the frequency of race and ethnicity reporting; and 2) the racial and ethnic distribution of participants in interventional clinical trials enrolling persons with hemophilia (PwH).
Methods: In this cross-sectional study, the clinicaltrials.gov database was queried in April 2023. All studies with “hemophilia” as the condition, coded as “interventional” and with “results” were selected (n=157). The proportion of trials reporting race/ethnicity and the distribution of race/ethnicity based on the US Office of Budget Management Categories (OBM) was computed according to trial characteristics. The racial/ethnic distribution of trial participants (observed) was compared to what would be expected based on United States (US) hemophilia treatment center (HTC) data and census data from four countries (Brazil, Canada, United Kingdom, and US) with observed-to-expected ratios (O/E) and 95% Confidence Intervals (CI).
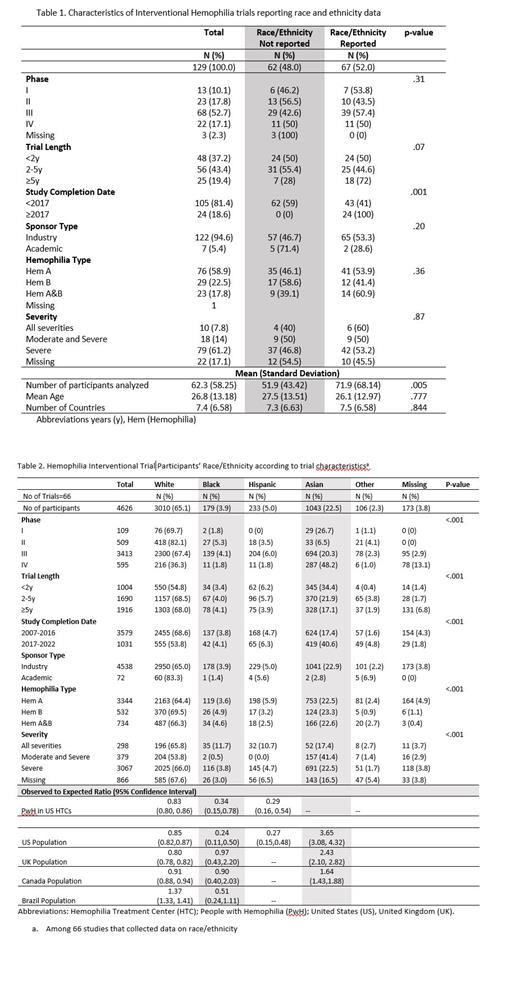
Results: Of the 157 trials identified, 28 were excluded because they were prematurely terminated. Of the 129 completed trials, the completion year ranged from 2007 to 2022, the large majority were industry sponsored (94.6%), and included an average of 62 participants with an average age of 26.8 years. Overall, 52.0% (n=66) of trials reported race/ethnicity data, which increased from 41.0% before 2017 to 100% in 2017 or later (p-value=.001) (Table 1). Among the 66 trials that reported race/ethnicity data, 49 (74.2%) were conducted in two or more countries, and the most common sites included the US (n=42 trials, 63.6%), Italy (n=26, 39.4%), Poland (n=24, 36.3%), Germany (n=22, 23.3%), and France (n=22, 33.3%). In terms of race/ethnicity, 65.1% of trial participants were White, 22.6% were Asian, 5.0% were Hispanic, 3.9% were Black, 2.3% were other race/ethnicity, and 3.8% were missing data on race/ethnicity. The proportion of trial participants who were White was approximately 20% lower than the US HTC population (O/E=0.83, 95%CI 0.80, 0.86) and 10-20% lower than the total population of the US (O/E=0.85, 95%CI 0.82,0.87), United Kingdom (O/E=0.80, 95%CI 0.78, 0.82), and Canada (O/E=0.91, 95% CI 0.88, 0.94) (Table 2). The proportion of trial participants who were Black or Hispanic was approximately 75% lower than the US HTC and the US total population. The percentage of trial participants who were Asian was 1.6 to 3 times higher than general population in the US, Canada, and UK (Table 2). We were unable to assess the number of racial/ethnic minorities enrolled in the US as these detailed data are not reported in clinicaltrials.gov. Another limitation is that ethnicity definitions vary across country's censuses.
Conclusions: Our study found that less than half of interventional hemophilia trials conducted before the 2017 FDA requirement to collect data on race/ethnicity did so, whereas all trials conducted in 2017 and later reported race/ethnic data. Compared to the US HTC and total population, Black and Hispanic people with hemophilia are especially underrepresented in hemophilia interventional trials. In 2020, the US FDA provided guidance on eligibility criteria, enrollment practices, and trial designs to increase diversity in underrepresented racial and ethnic participants. Rare diseases, including hemophilia, require additional efforts to improve diversity in clinical trials given the smaller patient population (The FDA, 2020). These efforts may include engaging patient advocacy groups in the trials process and leveraging international HTC networks.
Disclosures
Tran:Bayer, Biomarin, HEMA Biologics, Genentech, Takeda, and UniQure. He has been a consultant for Bayer and Novo Nordisk: Other: medical advisory boards. Kempton:Pfizer: Honoraria; Spark Therapuetics: Honoraria; Genetech: Honoraria; BioMarin: Honoraria; Sanofi US: Honoraria; Takeda: Honoraria.